BACKGROUND
At first plastics were welcome as blessings. Specifically, their durability, high strength per weight and low cost have made them the ideal packaging materials. However, the last seven decades have proved, those same attributes combined with improper waste management, led to a plastic pollution problem. An estimated 6300 million metric tons (Mt) of plastics were generated from 1950 to 2015, of which only 9% was recycled and 12% incinerated. The remaining 79% was disposed to the landfills or environment, an important portion of which has been defragmented to micro/nanoplastics. With microplastics detectable in drinking water, ambient air, and human placenta, plastic pollution is at alarming levels. In mitigation of this threat, biodegradable polymers have given hope, but their high cost and off-spec properties limit their use.
SUMMARY OF TECHNOLOGY
Our research has developed a unique approach to deal with plastic pollution, in particular remediate the inadvertently disposed plastics in the environment. It involves specific polymer structures, which transform to highly biodegradable natural polyesters (e.g., polyhydroxyalkanoates) or polyamides via oxidation with singlet oxygen (1O2). Specifically, photooxidation occurs through reaction of photosensitized 1O2 with C─H bonds, adjacent to electronegative heteroatoms (or electron-withdrawing heterogroups) in the carbon chain. When the heteroatom is O or N, the insertion results in an ester or peptide linkage, respectively, which are active sites for biotic (enzymatic) hydrolysis.
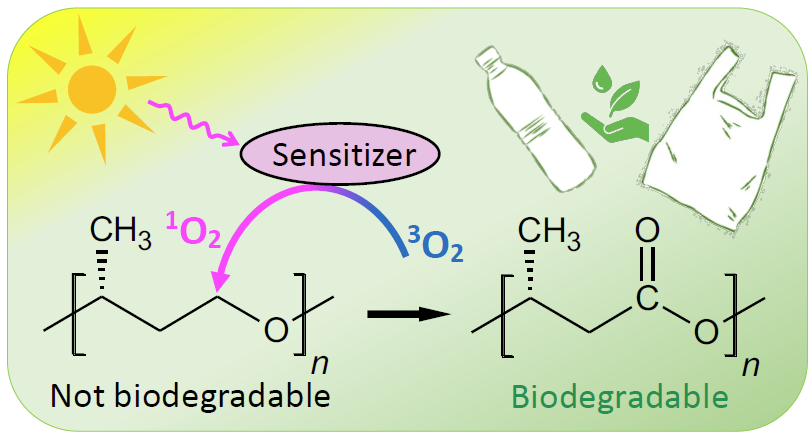
Therefore, specific polymer structures may be designed, which are meant to be not biodegradable originally but transform to a biodegradable structure (e.g., polyhydroxyalkanoates) under solar radiation after end of life. We estimate complete disintegration of a plastic water bottle could be reduced to about two years through 1O2-driven photodegradation in seawater and under AM1.5 solar radiation. Compared with biodegradable polymers, these new generation of green plastics are structurally less complex, with a less degree of structural and property deviations from the conventional commodity plastics. This aspect is expected to enable their manufacturability with conventional techniques. It also promises a significantly lower production cost. Additionally, the 1O2-driven photooxidation scheme, claimed here, has the potential to enable synthetic production of highly biodegradable natural polyesters at industrial scale, such as polyhydroxyalkanoates (PHAs, e.g., P3HB), for biomedical, pharmaceutical and agricultural applications. Current industrial production of PHAs involve expensive and low throughput steps of bacterial fermentation of sugars/lipids, extraction and purification, limiting their commercial potential.
POTENTIAL AREAS OF APPLICATION
- Recycling
- Manufacturing Biodegradable Plastic
STAGE OF DEVELOPMENT