BACKGROUND
Carbamate esters, also known as urethanes, are a key functional group in myriad medicinal and agricultural compounds, broadly functioning as hydrolytically and proteolytically stable peptide mimetics. Their end products such as antibiotics, cancer treatments, pesticides, and derived polyurethane materials bear markets valued well over 20 billion USD annually. Current means of production rely on either carbon monoxide (CO) or, to a far lesser extent, carbon dioxide (CO2) as its carbonyl C1 source. However, methods employing CO rely on burning fossil fuels and create toxic and cumbersome high energy molecules (e.g., chlorine and phosgene) which are critical to make the process exergonic. Current CO2 based methods require high amounts of energy and pressure in an autoclave due to the reaction’s endergonic nature and take days to produce low yields. There is a clear need for energy-efficient methods of producing these highly sought-after chemical group while reducing the amount of toxic intermediates.
SUMMARY OF TECHNOLOGY
Researchers at OSU have developed a mild and convenient method of CO2 aminoalkylation using visible light energy to drive the endergonic process- urethane synthesis (+25 kcal/mol at STP). Specifically, arylcyclohexenes play a key role, by converting the energy of blue photons into strain energy upon isomerization; the strain energy results in a dramatic increase in the basicity of the cycloalkene. This basicity results in the protonation of the strained cycloalkene by the in situ formed carbamic acid (a thermodynamically favored adduct), a reaction which occurs spontaneously at standard temperature and pressure. This results in the creation of an arylcyclohexyl urethane, which is conveniently converted into target urethane products via base catalysis by reacting it with the desired alcohol. This process amounts to a transurethanation and is favored by the reduction in steric bulk of the urethane. Making transurethanation to carbamate esters in which the ester is derived from a secondary or primary alcohol. The output of this process is arylcyclohexanol. Once trans-urethanation is completed, the original arylcyclohexenes can be regenerated simply by treatment with catalytic amounts of acid, which can be reused in the process in further rounds of reactions (see Figure 1). In total, the only stoichiometric by product to this process is the formation of a water molecule. The reaction takes place between 0 °C-RT, and is tolerant of water, low CO2 pressures, is functional group tolerant. This represents the first example of CO2 valorization using visible light as an indirect driving force by way of an “energy currency” of molecular strain, showing a potential to greatly advance current manufacturing methods of this important functional group.
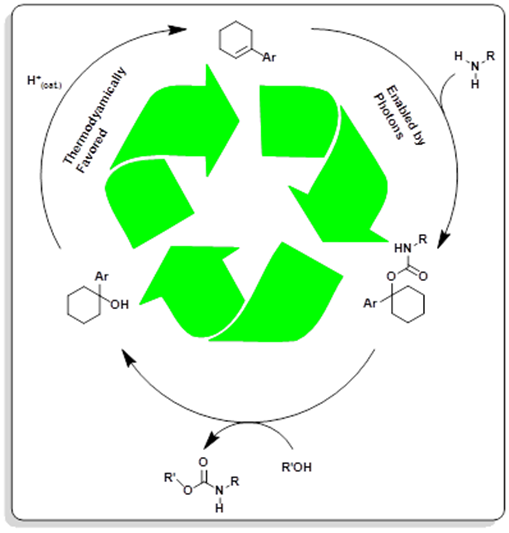 Step-wise regeneration of cyclohexene
Step-wise regeneration of cyclohexene
POTENTIAL AREAS OF APPLICATION
- Pharmaceutical production
- Agricultural industry
- Polyurethane-dependent industries (e.g., foam manufacturing)
MAIN ADVANTAGES
- Significantly safer than current urethane synthesis methods
- Reduces cost and toxicity of C1 sourcing
- Valorizes carbon dioxide
- Water-tolerant process
- Occurs near standard temperature and pressure
STAGE OF DEVELOPMENT